Summary Card
Overview
Chest wall reconstruction restores structural support, protects thoracic organs, preserves respiratory mechanics, and improves aesthetics, often after oncologic resection, trauma, or infection.
Chest Wall Anatomy
There are 3 main anatomical areas to be aware of in chest wall reconstruction: muscles, blood supply, and nerve innervation.
Principles of Chest Wall Reconstruction
Successful chest wall reconstruction requires adequate debridement followed by obliteration of thoracic dead space, skeletal stabilization, and soft tissue coverage.
Indications
Chest wall reconstruction is indicated for defects >5 cm, or when structural support, protection of intrathoracic contents, respiratory function, or aesthetics are compromised.
Assessment
The assessment of the chest wall defect can be simplified into defect characteristics (size, location, depth), surrounding tissue quality, and patient-specific factors, within a multidisciplinary framework.
Skeletal Reconstruction
Skeletal reconstruction materials must balance rigidity, malleability, and biocompatibility. Options include autologous grafts and alloplastic materials such as mesh, PTFE, titanium plates, and biologic scaffolds.
Soft Tissue Reconstruction
Main muscles for soft tissue reconstruction are pectoralis major, latissimus dorsi, and rectus abdominis. Omentum can also be used.
Primary Contributor: Dr Waruguru Wanjau, Educational Fellow.
Verified by thePlasticsFella ✅
Overview of Chest Wall Reconstruction
Chest wall reconstruction restores structural support, protects thoracic organs, preserves respiratory mechanics, and improves aesthetics, often after oncologic resection, trauma, or infection.
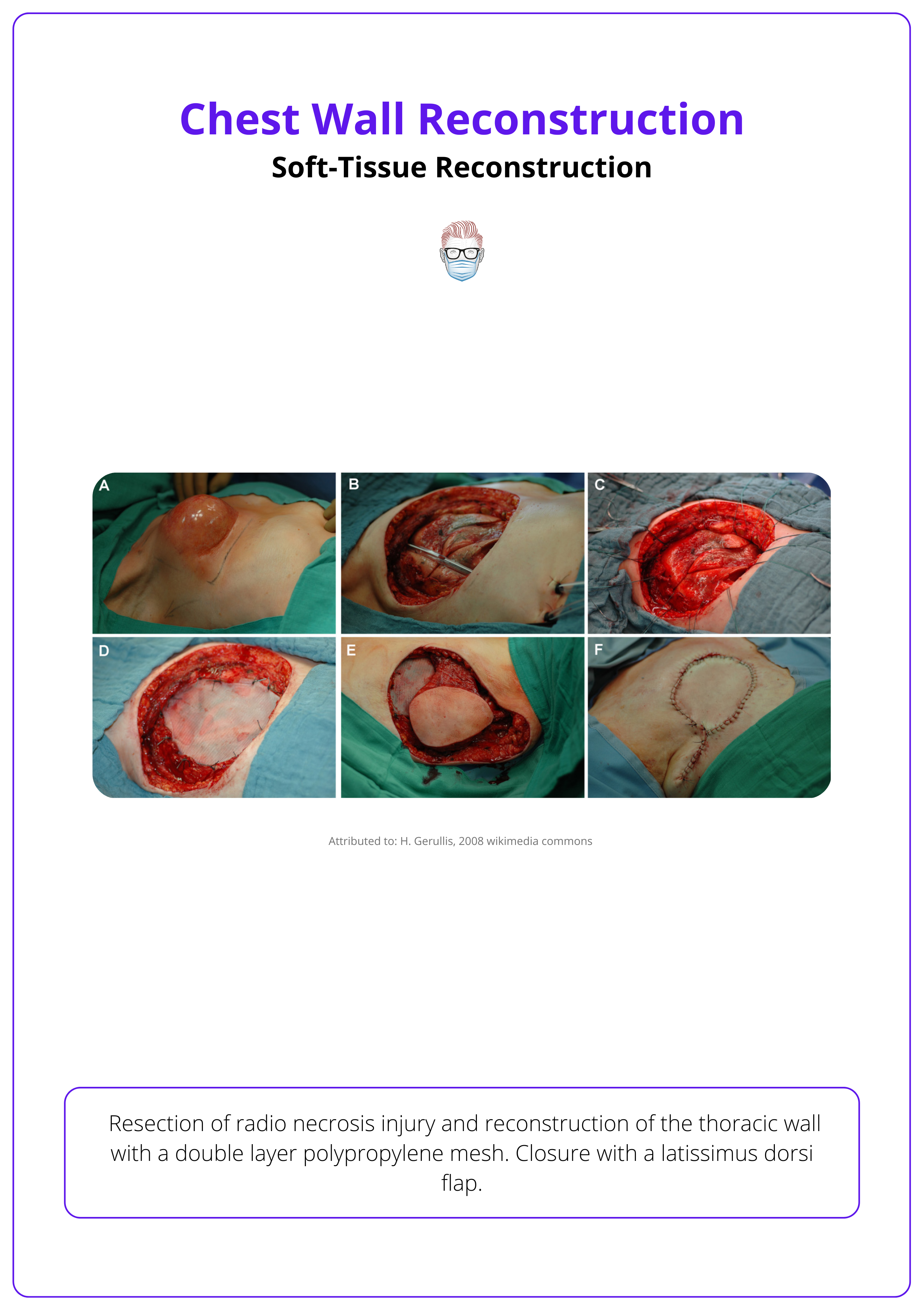
Chest wall reconstruction is a complex and multidisciplinary process required when the thoracic cage is compromised in structure or function. This can occur due to cancer resection, trauma, infection, congenital anomalies, or prior surgical complications.
Reconstruction aims to achieve four primary goals.
- Restore stability and support to the chest wall to prevent flail segments or paradoxical motion.
- Protect intrathoracic structures such as the lungs and mediastinum from external trauma or infection.
- Preserve respiratory mechanics, minimizing restrictive impairment.
- Provide soft-tissue coverage that is durable, vascularised, and resistant to infection, especially over exposed organs or prosthetic material.
Defect characteristics such as size, location, and depth guide reconstructive planning, along with patient comorbidities, prior treatment (e.g., radiation), and available reconstructive options. Flap selection is based on local anatomy, vascular pedicles, and donor site morbidity, while skeletal defects may require rigid reconstruction using prosthetic materials or titanium supports.
Early cases relied on muscle flaps such as the latissimus dorsi (Tansini, 1906), but modern approaches integrate advanced techniques including 3D printing, custom implants, and acellular dermal matrices. Successful outcomes rely on sound surgical planning, a biomimetic approach, and close collaboration with thoracic surgery, oncology, and intensive care teams.
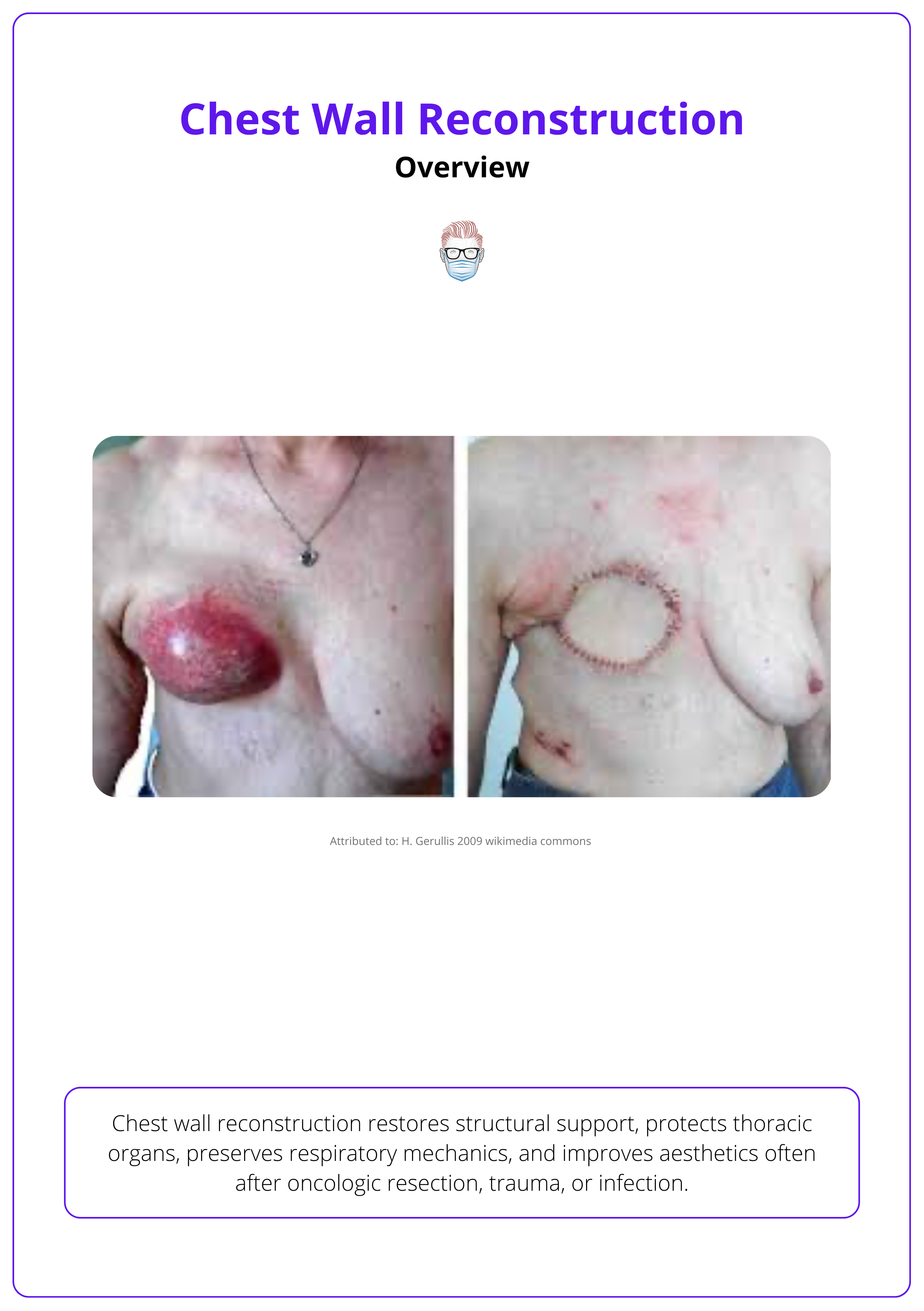
Pre- and post-operative macroscopic presentation is illustrated below.
Anatomy of the Chest Wall
Chest wall reconstruction relies on an understanding of three core anatomical elements: muscles, blood supply, and nerve innervation.
A full anatomical review is beyond scope, but a working knowledge of chest wall structures is essential for flap design and preserving respiratory function.
Musculature
Muscles are grouped based on their relation to the thoracic cage.
- Thoracic Cage Muscles: Intercostals, subcostals, and transversus thoracis support ventilation and structural integrity.
- Muscles Inserting onto the Cage: Pectoralis major/minor, serratus anterior, and scalenes are key for both function and reconstruction.
Respiratory roles include,
- Inspiratory: Diaphragm, scalenes, sternocleidomastoid.
- Expiratory: Abdominals, obliques.
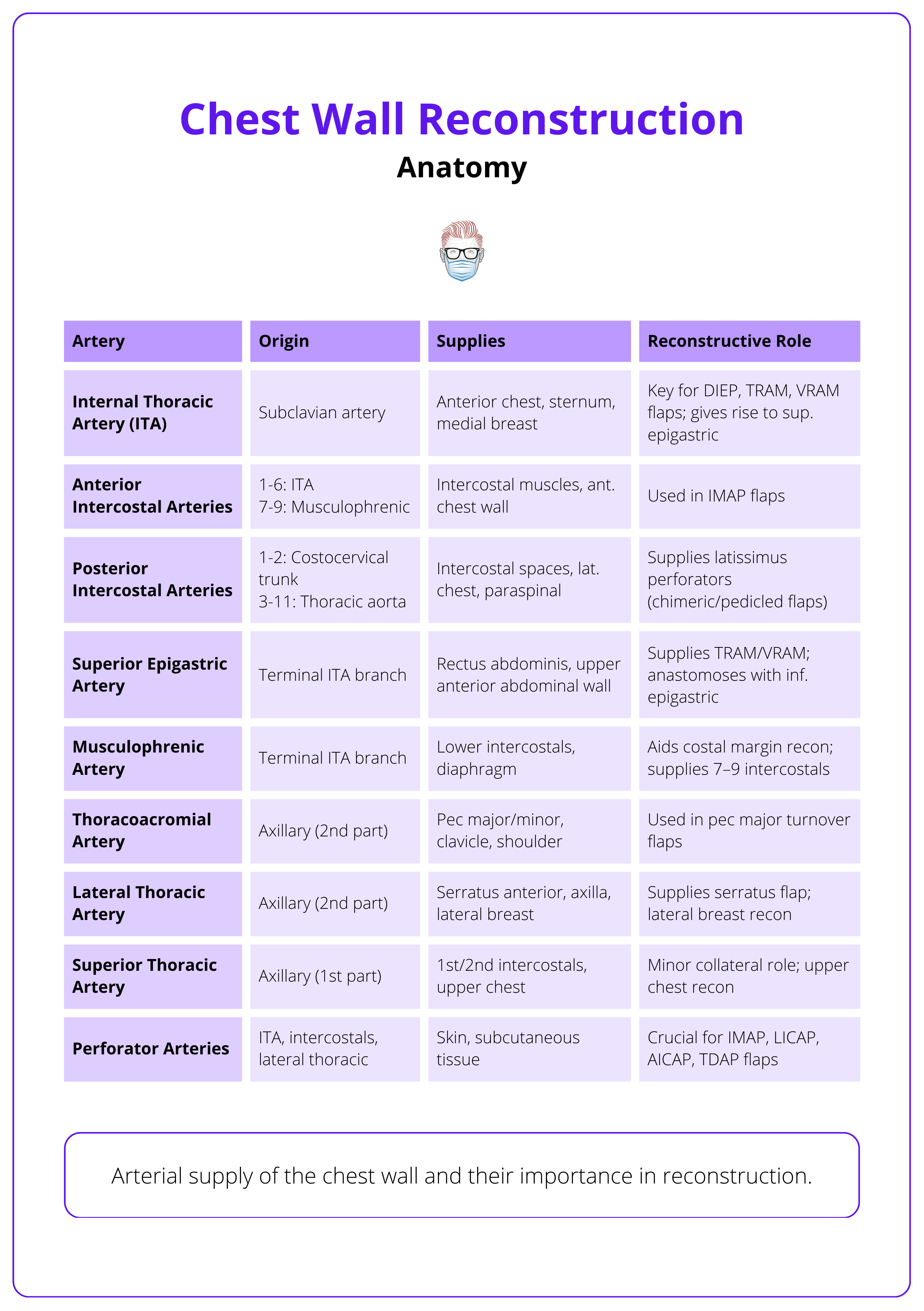
Vascular Supply
- Arterial: Primarily from internal thoracic arteries; with input from posterior intercostals, lateral thoracic, and thoracoacromial branches.
- Venous: Mirrors arterial pathways; posterior drainage via the azygous system.
Nerve Supply
- Intercostal nerves (T1-T11): Innervate muscles and overlying skin; crucial to preserve when possible.
The arterial supply of the chest wall and their reconstructive role is summarised below.
Principles of Chest Wall Reconstruction
Effective chest wall reconstruction depends on thorough debridement, elimination of dead space, skeletal stabilization, and soft tissue coverage.
The aim of chest wall reconstruction is to restore both function and form—achieving biomimesis or anatomical mimicry of the native chest wall (Rocco, 2010). Restoring skeletal integrity prevents paradoxical movement, protects intrathoracic contents, and supports respiratory mechanics.
This is guided by fundamental reconstructive principles, each tailored to defect size, location, and patient physiology.
- Anatomical and Structural Restoration: Respect native planes and geometry. Skeletal defects, especially anterior or large, require rigid or semi-rigid fixation to preserve chest mechanics and respiratory function.
- Tissue Coverage and Organ Protection: Well-vascularised flaps (preferably locoregional) provide soft tissue coverage, eliminate dead space, and protect vital organs. Avoid rigidity that compromises lung or diaphragm movement.
- Precision and Planning: Optimal outcomes depend on meticulous technique, multidisciplinary coordination, and thoughtful material selection, balancing function, aesthetics, and complication risk (Anikin, 2020). Advanced tools like 3D printing and NPWT can support complex reconstructions.
In 1906, Tansini pioneered chest wall reconstruction using a pedicled latissimus dorsi flap to repair an anterior thoracic defect (Tansini, 1906).
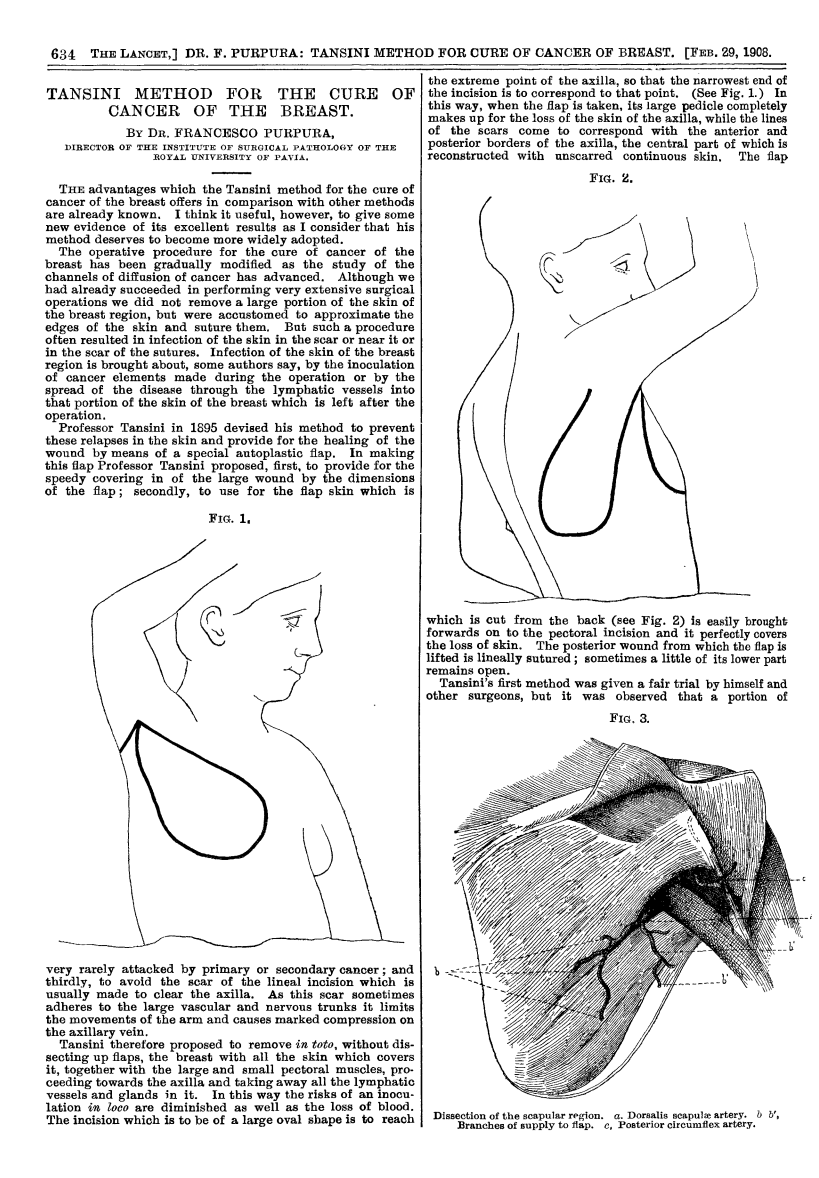

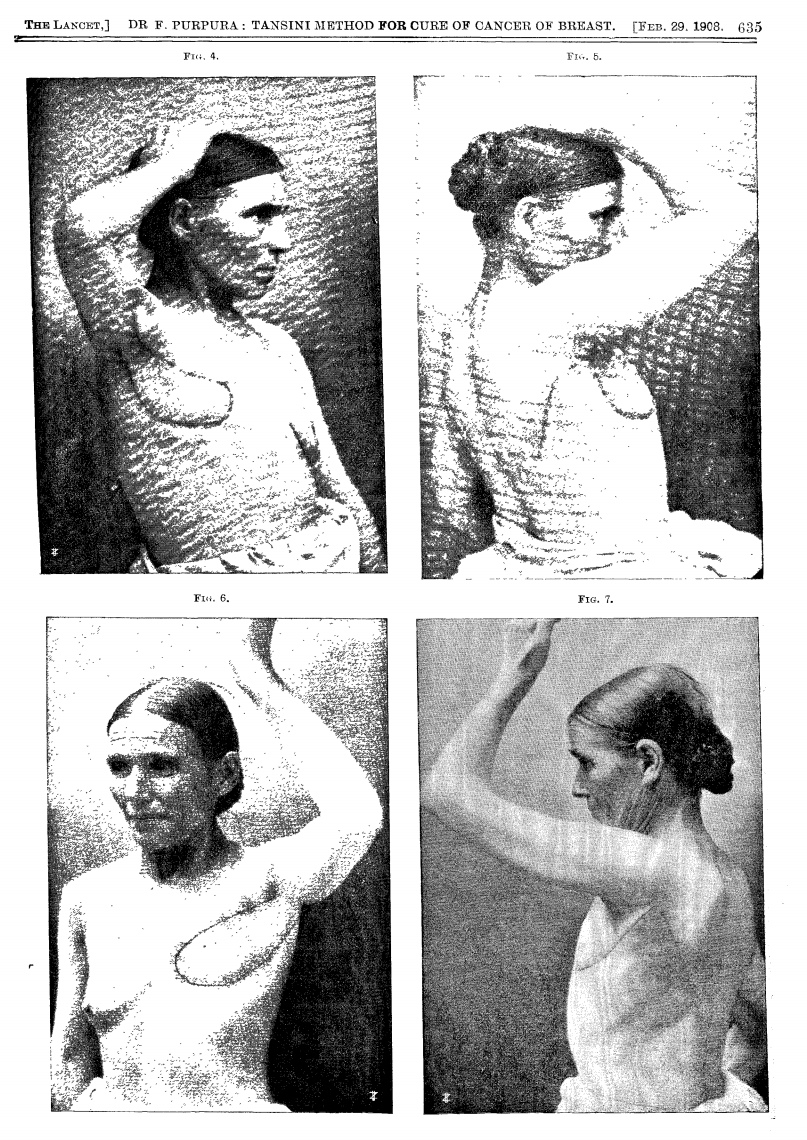
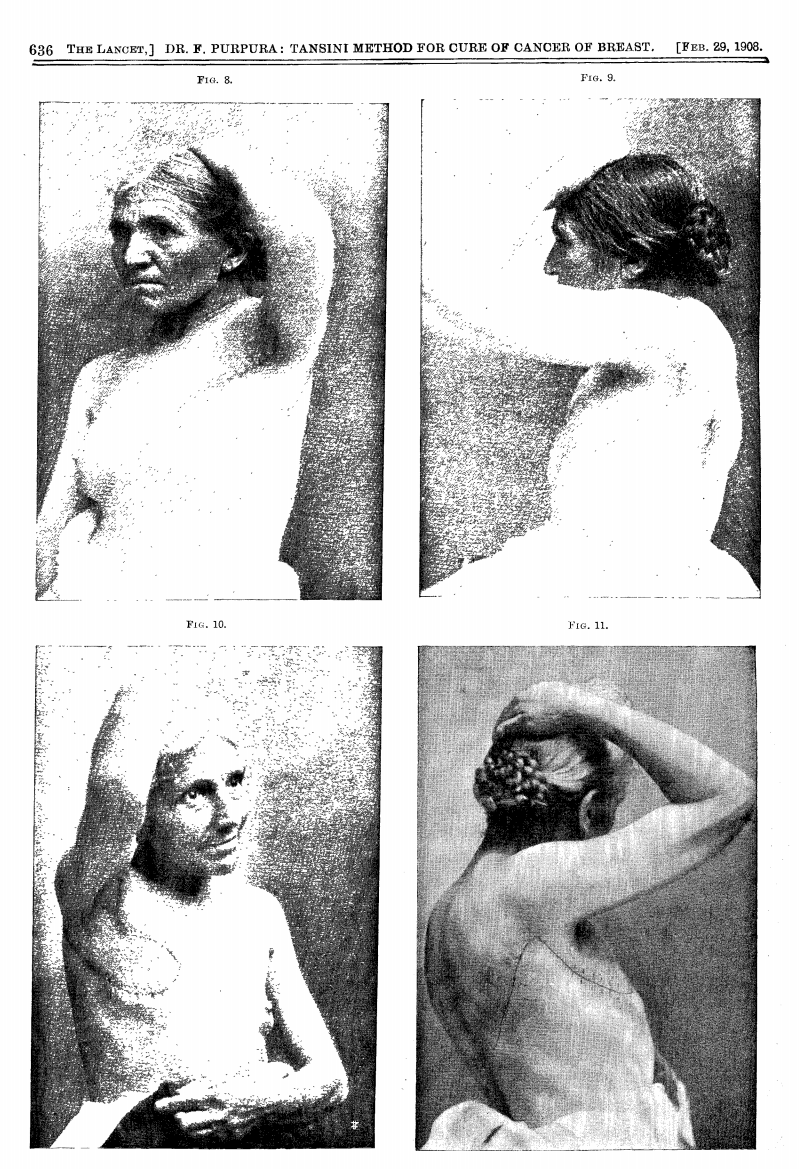
1908 Lancet Publication translating the work of Tansini
Indications of Chest Wall Reconstruction
Chest wall reconstruction is indicated for defects >5 cm, or when structural support, protection of intrathoracic contents, respiratory function, or aesthetics are compromised due to oncologic, traumatic, congenital, or iatrogenic causes.
Reconstruction is required when the thoracic cage loses its structural integrity, stability, or soft tissue coverage, regardless of the underlying cause. These defects can arise from resection, trauma, infection, or prior surgery, and often demand a tailored approach based on anatomical and functional needs.
General Indications
- Defect Size >5 cm: Often requires skeletal reconstruction, particularly if full-thickness (Anikin, 2020).
- Loss of ≥4 Ribs: Associated with paradoxical chest movement and risk of lung herniation; rigid support may be required (Anikin, 2020).
- Sternal Resection: Especially involving the manubrium; reconstruction prevents anterior instability.
- Exposed Lung, Pleura, or Mediastinum: Necessitates robust flap coverage to protect vital organs.
The indications for stabilisation are summarised below.

Anterolateral defects more frequently require reconstruction due to the lack of inherent bony support in this region (Anikin, 2020).
Individualised Decision-Making
Indications should not be applied rigidly. Surgical judgment, patient factors, and resource availability all play a role. Expert variation in practice includes,
- Apicoposterior defects may be left unreconstructed if the scapula provides natural support (Deschamps, 1999).
- Cartilage-level resections (4–6 ribs) are sometimes managed conservatively without reconstruction (Deschamps, 1999).
- Some surgeons reconstruct nearly all defects to pre-empt instability or patient dissatisfaction (Hughes, 1997).
Radiation-induced fibrosis may provide sufficient rigidity, making stabilisation unnecessary in some large defects (Losken, 2004).
Assessment of Chest Wall Defects
Assessment should focus on defect characteristics, surrounding tissue condition, and patient-specific factors, ideally within a multidisciplinary team setting.
A thorough evaluation informs timing, feasibility, and the overall strategy for reconstruction. It determines the need for skeletal support, flap selection, and perioperative planning.
Defect: Size, Location, and Depth
The reconstruction decision algorithm is strongly influenced by the size, location, and depth of the chest wall defect.
- Size: Large defects (>5 cm) often require skeletal reconstruction, especially if full thickness.
- Location
- Anterolateral: Greater impact on respiratory mechanics due to lack of sternal or spinal support; structural restoration usually necessary.
- Anterior: Suitable for pectoralis major or latissimus dorsi flaps.
- Superoposterior: Often supported by the scapula; may not require skeletal stabilization.
- Depth
- Partial-Thickness: May only require skin grafts.
- Full-Thickness: Usually requires both skeletal and soft tissue reconstruction.
Surrounding Tissue
- Infection may necessitate staged reconstruction with initial debridement.
- Radiation impairs vascularity—flaps should be sourced from non-irradiated areas.
- Oncologic resection requires clear margins and reliable coverage.
- Dead space must be assessed and obliterated, especially when vital structures are exposed.
Irradiated tissues are stiffer, but less likely to have paradoxical chest movements post-resection.
Patient Factors
- Assess overall suitability for surgery, cardiopulmonary and nutritional status are key.
- Comorbidities like diabetes or hypertension must be optimised preoperatively.
- Smoking cessation should occur at least 4 weeks before surgery.
- Review surgical history, including prior internal mammary artery harvest.
- Factor in adjuvant therapies, especially radiotherapy, which impacts flap selection.
The use of flaps for chest wall reconstruction is illustrated below.
After sternal resection, muscle flaps may shift reliance to abdominal breathing. This is well tolerated by most, but impactful for patients with pre-existing pulmonary disease (Netcher, 2009).
Skeletal Reconstruction Options
Skeletal reconstruction materials must balance rigidity, malleability, and biocompatibility. Options include autologous grafts and alloplastic materials such as mesh, PTFE, titanium plates, and biologic scaffolds.
The goal is to restore chest wall rigidity, prevent paradoxical motion, and protect intrathoracic organs. The ideal material should be strong enough to stabilise the defect, yet flexible enough to contour to complex anatomy. It must also be biocompatible, radiolucent, and inert to minimise complications (Losken, 2004).
There is no universal consensus on the best material (Momeni, 2016) Selection depends on,
- Defect size, location, and contamination
- Surgeon preference and experience
- Institutional resources (Momeni, 2016)
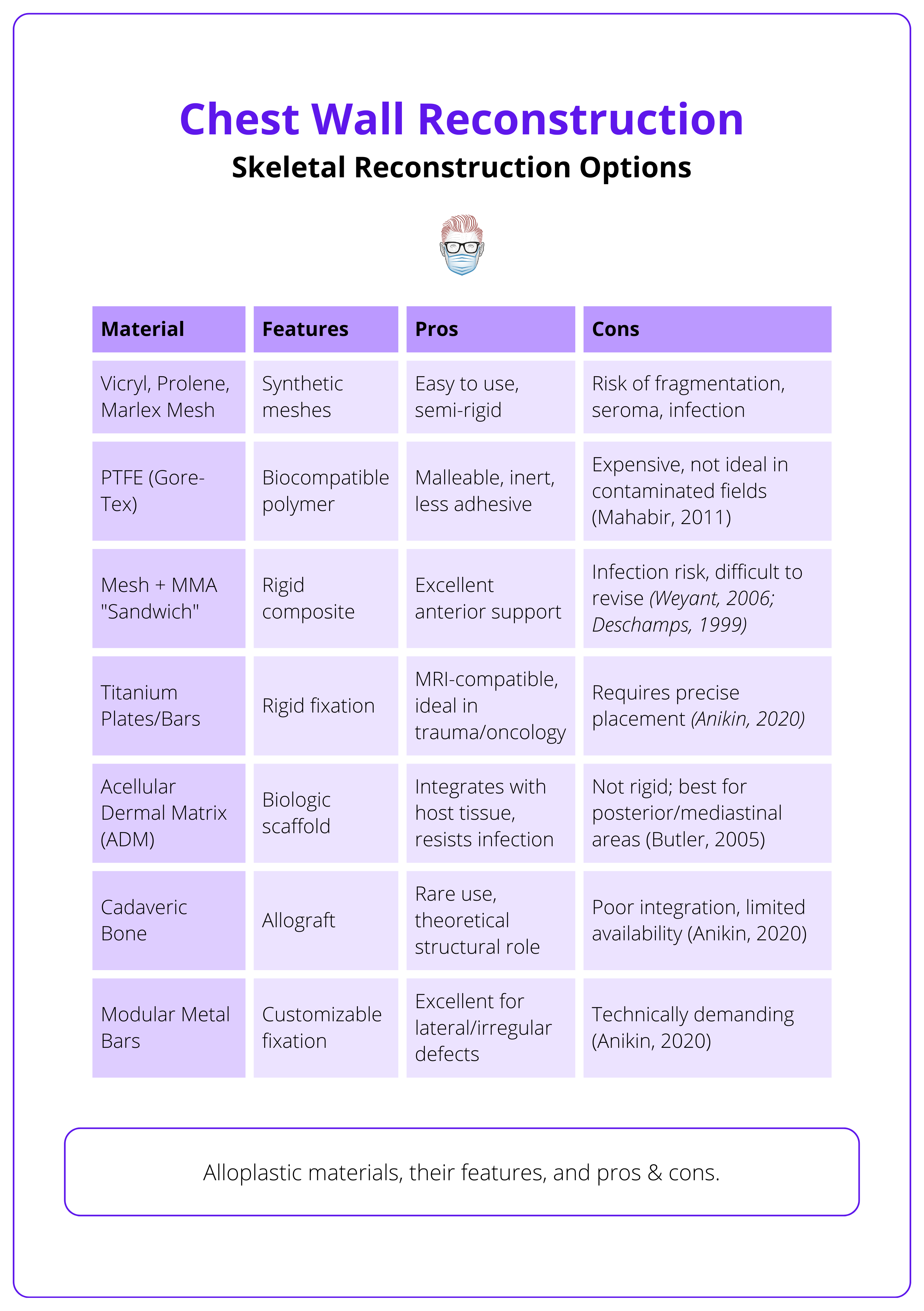
Alloplastic Materials
More commonly used due to their versatility and predictable performance. Each has specific indications and limitations, as summarised in the table below.
Autologous Materials
Now rarely used, autologous options include,
- Split rib grafts, iliac crest, fibula, and fascia.
- Limitations: increased donor-site morbidity, limited shaping, and unpredictable resorption.
ADM does not create a rigid reconstruction but offers enough support to prevent paradoxical motion (Butler, 2005).
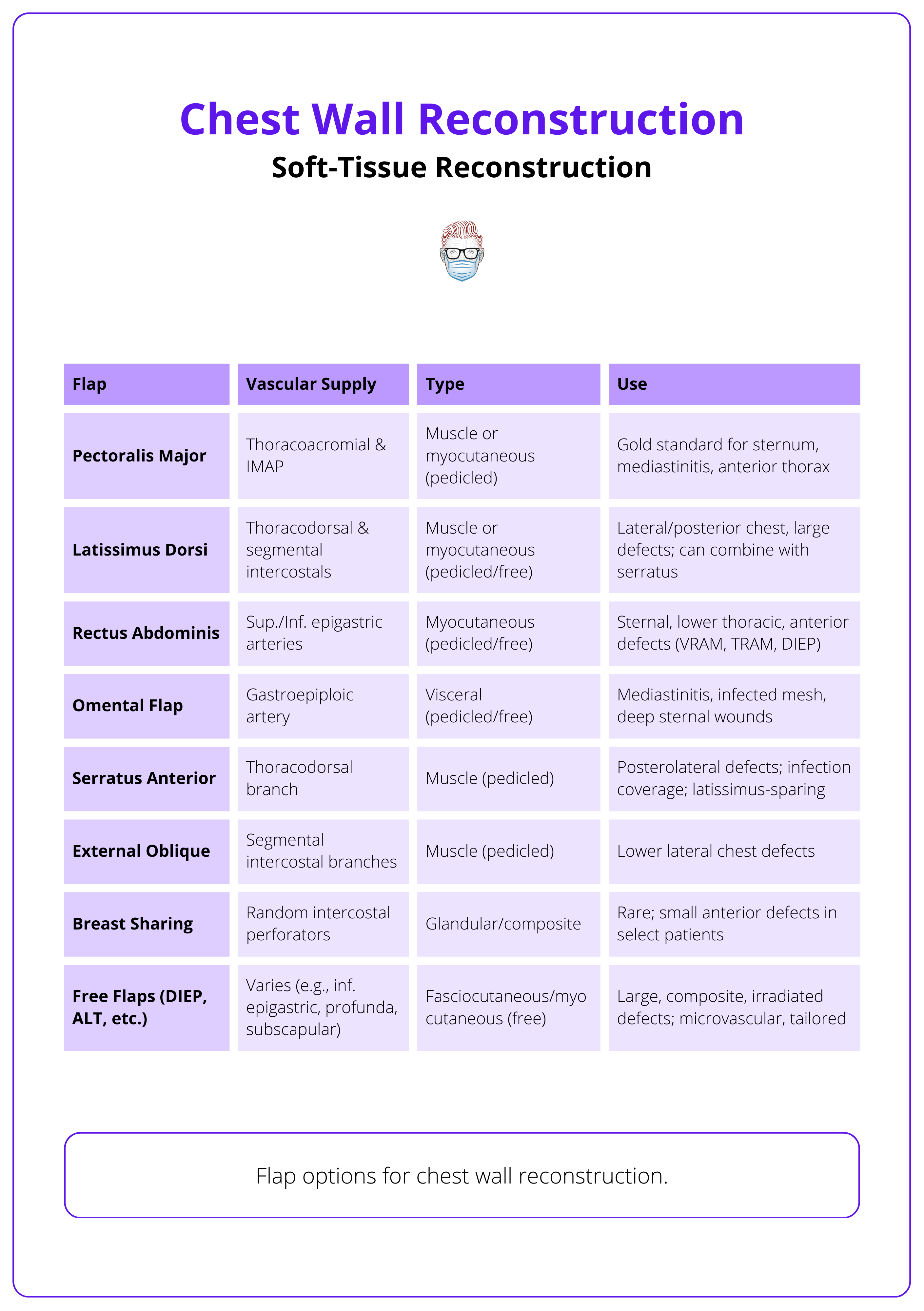
Soft-Tissue Reconstruction of the Chest
Main muscles for soft tissue reconstruction are pectoralis major, latissimus dorsi, and rectus abdominis. Omentum can also be used.
Soft tissue reconstruction aims to provide robust, vascularised coverage of vital thoracic structures, particularly when skeletal reconstruction is not sufficient to restore protection or contour. Flap selection depends on defect location, size, prior surgeries, and available donor sites.
Commonly used flaps include,
- Pectoralis Major Flap
- Ideal for anterior defects, especially post-sternotomy.
- Reliable vascularity via thoracoacromial vessels.
- Can be advanced, turned over, or islanded.
- Latissimus Dorsi Flap
- Versatile muscle, suitable for anterolateral or posterior defects.
- Large surface area, long pedicle (thoracodorsal vessels).
- Can be used as a muscle or musculocutaneous flap.
- Rectus Abdominis Flap
- Pedicled superiorly via superior epigastric artery.
- Suitable for lower anterior chest wall defects.
- Can be raised even if internal mammary arteries are sacrificed (via eighth subcostal artery) (Fernando 1988).
- External Oblique and Serratus Anterior Flaps
- Useful for lateral and posterolateral defects.
- External oblique has broad surface but less bulk.
- Serratus anterior is thin, pliable, and based on thoracodorsal vessels.
- Omental Flap
- Excellent vascularity and immune properties.
- Best for infected or irradiated fields, mediastinal coverage, or irregular dead spaces.
- Harvested via laparotomy or laparoscopy.
The use of these flaps for chest wall reconstruction is illustrated below.
The rectus abdominis muscle can still be used even if the internal mammary artery has been taken, thanks to its collateral supply from the eighth subcostal artery (Fernando, 1988).
Further Reading
- Rocco G. Chest wall surgery. Thorac Surg Clin 20:xiii, 2010 2. Rocco G: Overview on current and future materials for chest wall reconstruction. Thorac Surg Clin 20:559-562, 2010
- Din AM, Evans GR. Chest wall reconstruction. In: McCarthy JB, Galiano RD, Boutros S, editors. Current therapy in plastic surgery. Philadelphia: Saunders; 2006. p. 362
- Seder CW, Rocco G. Chest wall reconstruction after extended resection. J Thorac Dis 2016;8:S863-S871.
- Ferraro P, Cugno S, Liberman M, et al. Principles of chest wall resection and reconstruction. Thorac Surg Clin 2010;20:465-73.
- Mahabir RC, Butler CE. Stabilization of the chest wall: autologous and alloplastic reconstructions. Semin Plast Surg 2011;25:34-42.
- Deschamps C, Tirnaksiz BM, Darbandi R, et al. Early and long-term results of prosthetic chest wall reconstruction. J Thorac Cardiovasc Surg 1999;117:588-91. 13.
- Arnold PG, Pairolero PC. Chest wall reconstructions: an account of 500 consecutive cases. Plast Reconstr Surg 1996; 98(5):804
- Rocco G. Chest wall resection and reconstruction according to the principles of biomimesis. Semin Thorac Cardiovasc Surg 2011;23:307-13.
- Losken A, Thourani VH, Carlson GW, Jones GE, Culbertson JH, Miller JI, Mansour KA. A reconstructive algorithm for plastic surgery following extensive chest wall resection. Br J Plast Surg. 2004 Jun;57(4):295-302. doi: 10.1016/j.bjps.2004.02.004. PMID: 15145731.
- Bostwick J, Nahai F, Wallace JG, et al. Sixty latissimus dorsi flaps. Plast Reconstr Surg 1979;63:31.
- Tansini I. Sopra il mio processo di amputazione della mammella. Gazz Med Ital Torino 1906;57:141 [in Italian]
- Deschamps C, Timaksiz BM, Darbandi R, et al. Early and long-term results of prosthetic chest wall reconstruction. J Thorac Cardiovasc Surg 1999;117:588.
- Weyant MJ, Bains MS, Venkatraman E, et al. Results of chest wall resection and reconstruction with and without rigid prosthesis. Ann Thorac Surg 2006;81:279-85.
- Losken A, Thourani VH, Carlson GW, Jones GE, Culbertson JH, Miller JI, Mansour KA. A reconstructive algorithm for plastic surgery following extensive chest wall resection. Br J Plast Surg. 2004 Jun;57(4):295-302. doi: 10.1016/j.bjps.2004.02.004. PMID: 15145731.
- Jones G, Jurkiewicz MJ, Bostwick J, et al. Management of the infected median sternotomy wound with muscle flaps. The Emory 20-year experience. Ann Surg 1997;225:766.
- Nahai F, Morales L Jr, Bone DK, et al. Pectoralis major muscle turnover flaps for closure of the infected sternotomy wound with preservation of form and function. Plast Reconstr Surg 1982;70:471
- Jurkiewicz MJ, Arnold PG. The omentum: an account of its use in reconstruction of the chest wall. Ann Surg 1977;185:548.
- Hughes KC, Henry MJ, Turner J, Manders EK. Design of the cyclops flap for chest-wall reconstruction. Plast Reconstr Surg. 1997 Oct;100(5):1146-51; discussion 1152. doi: 10.1097/00006534-199710000-00011. PMID: 9326775.
- Bogossian N. A system for accessing our valuable human resources. Plast Reconstr Surg. 1996 Jan;97(1):253. doi: 10.1097/00006534-199601000-00055. PMID: 8532796.
- Moschella F, Cordova A. A new extended external oblique musculocutaneous flap for reconstruction of large chest-wall defects. Plast Reconstr Surg. 1999 Apr;103(5):1378-85. doi: 10.1097/00006534-199904050-00006. PMID: 10190434.
- Anikin, V., 2020. Principles of complex chest wall reconstruction. Shanghai Chest, 4.
- Kerrigan CL, Daniel RK. The intercostal flap: an anatomical and hemodynamic approach. Ann Plast Surg. 1979 May;2(5):411-21. PMID: 543605.
- Clemens MW, Evans KK, Mardini S, Arnold PG. Introduction to chest wall reconstruction: anatomy and physiology of the chest and indications for chest wall reconstruction. Semin Plast Surg. 2011 Feb;25(1):5-15. doi: 10.1055/s-0031-1275166. PMID: 22294938; PMCID: PMC3140236.
- Minabe T, Harii K. Dorsal intercostal artery perforator flap: anatomical study and clinical applications. Plast Reconstr Surg. 2007 Sep;120(3):681-689. doi: 10.1097/01.prs.0000270309.33069.e5. PMID: 17700119.
- Fernando B, Muszynski C, Mustoe T. Closure of a sternal defect with the rectus abdominis muscle after sacrifice of both internal mammary arteries. Ann Plast Surg. 1988 Nov;21(5):468-71. doi: 10.1097/00000637-198811000-00013. PMID: 2906793.
- Momeni A, Kovach SJ. Important considerations in chest wall reconstruction. J Surg Oncol. 2016 Jun;113(8):913-22. doi: 10.1002/jso.24216. Epub 2016 Mar 9. PMID: 26969557.
- Netscher DT, Baumholtz MA. Chest reconstruction: I. Anterior and anterolateral chest wall and wounds affecting respiratory function. Plast Reconstr Surg. 2009 Nov;124(5):240e-252e. doi: 10.1097/PRS.0b013e3181b98c9c. PMID: 20009799.
- Deschamps C, Tirnaksiz BM, Darbandi R, Trastek VF, Allen MS, Miller DL, Arnold PG, Pairolero PC. Early and long-term results of prosthetic chest wall reconstruction. J Thorac Cardiovasc Surg. 1999 Mar;117(3):588-91; discussion 591-2. doi: 10.1016/s0022-5223(99)70339-9. PMID: 10047664.
- le Roux BT, Shama DM. Resection of tumors of the chest wall. Curr Probl Surg. 1983 Jun;20(6):345-86. doi: 10.1016/s0011-3840(83)80007-0. PMID: 6851629.
- Losken A, Thourani VH, Carlson GW, Jones GE, Culbertson JH, Miller JI, Mansour KA. A reconstructive algorithm for plastic surgery following extensive chest wall resection. Br J Plast Surg. 2004 Jun;57(4):295-302. doi: 10.1016/j.bjps.2004.02.004. PMID: 15145731.
- Butler CE, Langstein HN, Kronowitz SJ. Pelvic, abdominal, and chest wall reconstruction with AlloDerm in patients at increased risk for mesh-related complications. Plast Reconstr Surg. 2005 Oct;116(5):1263-75; discussion 1276-7. doi: 10.1097/01.prs.0000181692.71901.bd. PMID: 16217466.
- Mahabir RC, Butler CE. Stabilization of the chest wall: autologous and alloplastic reconstructions. Semin Plast Surg. 2011 Feb;25(1):34-42. doi: 10.1055/s-0031-1275169. PMID: 22294941; PMCID: PMC3140239.
- Garcia VF, Seyfer AE, Graeber GM. Reconstruction of congenital chest-wall deformities. Surg Clin North Am. 1989 Oct;69(5):1103-18. doi: 10.1016/s0039-6109(16)44941-8. PMID: 2551052.
- Corkum JP, Garvey PB, Baumann DP, Abraham J, Liu J, Hofstetter W, Butler CE, Clemens MW. Reconstruction of massive chest wall defects: A 20-year experience. J Plast Reconstr Aesthet Surg. 2020 Jun;73(6):1091-1098. doi: 10.1016/j.bjps.2020.02.010. Epub 2020 Feb 17. PMID: 32269009.