Summary Card
Overview
An angiosome is a three‑dimensional unit of tissue nourished by one artery and vein. Knowing where these territories begin and end allows surgeons to choose flaps with reliable perfusion and to avoid necrosis and donor‑site morbidity.
Vascular Territories and Regional Anatomy
Angiosomes are subdivided into anatomical, dynamic, and potential territories, representing progressively wider zones of perfusion as choke vessels open and collateral flow develops.
Choke Vessels and Delay Phenomenon
Choke vessels are narrow arteries and veins linking adjacent angiosomes. Over several days, they enlarge and transform into true anastomoses, thereby enlarging the functional territory. Harnessing their biology through delay procedures reduces necrosis and expands reconstructive options.
Clinical Applications
Angiosome mapping informs every stage of flap surgery from design through perforator selection to intra‑operative decision‑making. Thereby improving outcomes and guiding targeted revascularisation.
Benefits and Limitations
Angiosome‑based planning reduces necrosis, improves aesthetics, and guides targeted revascularisation, yet its predictive value is limited by individual vascular variability, comorbidities, and the need for time‑dependent dilation.
Verified by thePlasticsFella ✅
Overview of Angiosomes
An angiosome is a three‑dimensional unit of tissue nourished by one artery and vein. Knowing where these territories begin and end allows surgeons to choose flaps with reliable perfusion and to avoid necrosis and donor‑site morbidity.
Modern reconstructive microsurgery relies on transferring vascularised tissue rather than avascular grafts. Because angiosome-based flaps bring their own arterial and venous supply, they remain viable even in compromised wound beds.
The concept, first mapped by Taylor and Palmer (1987) and built on the anatomical studies of Manchot and Salmon, provides a vascular “atlas” for flap design. By defining the limits of reliable perfusion, surgeons can plan reconstructions with predictable survival and contour.
Core Principles
Angiosomes give a predictable map of the body’s vascular territories, each supplied by one source artery-vein pair. Their interconnections through choke vessels and true anastomoses explain flap reliability and the potential for perfusion beyond strict anatomical borders.
Every angiosome is composed of smaller perforasomes, each supplied by an individual perforator. Recognising these subdivisions is central to perforator-flap surgery, guiding both pre-operative imaging and intra-operative dissection.
Perforator Types
- A perforator is a block of skin, fat, fascia, muscle, or bone supplied by a single source artery and vein. Boundaries are defined by choke vessels or true anastomoses.
- Perforator Types
- Direct Cutaneous: Arise straight to the skin.
- Septocutaneous: Travel through intermuscular septa.
- Musculocutaneous: Passes through muscle before reaching the skin.
- Functional Angiosome: The tissue volume that can be safely perfused by a source vessel. It expands where true anastomoses link adjacent territories, but is smaller where only choke vessels connect them.
Angiosome Principles (Taylor & Palmer, 1987)
- Territorial Definition: Angiosomes define flap boundaries; adjacent zones meet at choke vessels or anastomoses.
- Connective-Tissue Pathways: Source vessels follow intermuscular septa and fascial planes, forming predictable harvest routes.
- Law of Equilibrium: When one artery is small, its neighbour enlarges to compensate.
- Continuity and Neurovascular Relationships: Arteries, veins, and nerves travel together from fixed to mobile points, maintaining continuity despite variable origins.
Flap Design Principles
Effective angiosome-based reconstruction requires precise geometry and meticulous technique.
- Length-to-Width Ratio: Keep random-pattern flaps ≤3:1 to reduce distal necrosis.
- Orientation & Tension: Align along the source vessel. Avoid twisting or kinking to prevent venous congestion.
- Respect Natural Boundaries: Plan incisions along angiosome or aesthetic unit borders for optimal contour.
- Tissue Handling: Elevate suprafascially where possible; preserve perforasomes and avoid over-skeletonising vessels to maintain perfusion.
Vascular Territories and Regional Anatomy
Angiosomes are subdivided into anatomical, dynamic, and potential territories, representing progressively wider zones of perfusion as choke vessels open and collateral flow develops.
For practical planning, surgeons distinguish the reliably perfused core tissue from adjacent zones that might be recruited after delay. Awareness of these layers and of perfusion gradients across different body regions allows safe harvest and minimises complications.
Territories of an Angiosome
For surgical planning, it is crucial to distinguish the core perfused zone from areas that can be recruited over time.
- Anatomical Territory: Directly supplied by the source artery’s perforators and reliably perfused without delay.
- Dynamic Territory: Perfused after choke vessels dilate during flap elevation or surgical delay, represents a time-dependent expansion of the core.
- Potential Territory: Becomes viable only after deliberate delay procedures, when choke vessels remodel into true anastomoses, useful in large composite or extended flaps.
Pre-operative imaging (e.g. indocyanine green angiography) and patient factors such as vascular disease, prior surgery, or smoking history help determine how much of the dynamic or potential territories can safely be included.
Foot and Ankle Angiosomes
The foot and ankle receive blood from three main arteries: The anterior tibial (ATA), posterior tibial (PTA), and peroneal arteries, which together form six angiosomes.
- Dorsalis Pedis (ATA): dorsum of the foot and extensor tendons.
- Medial Plantar (PTA): instep and medial forefoot.
- Lateral Plantar (PTA): lateral midfoot and forefoot.
- Calcaneal Branch (PTA): heel pad.
- Anterior Perforating and Calcaneal Branches (peroneal): lateral ankle, heel, and Achilles region.
Adjacent angiosomes are connected by arterial collaterals that can redirect flow when one territory is compromised. However, choke vessels open slowly, particularly in ischaemic limbs, underscoring the value of delay techniques or staged reconstructions in compromised vascular beds.
Abdominal Perfusion Zones (Hartrampf Classification)
In breast reconstruction, the Hartrampf zones describe perfusion across the lower abdominal flap based on the deep inferior epigastric artery (DIEA).
- Zone I: Ipsilateral and central - best perfused, forming the flap’s core.
- Zone II: Contralateral medial - less consistent, often dependent on crossover flow.
- Zone III: Ipsilateral lateral - may be better perfused than Zone II when lateral perforators dominate.
- Zone IV: Contralateral lateral - least perfused, often discarded or deepithelialised.
Subsequent refinements by Holm et al. and Saint-Cyr demonstrated that perfusion depends more on individual perforator dominance than on static zone boundaries.
This evolving understanding aligns with modern interpretations discussed in Zones of Perfusion by The Plastics Fella, which highlights how imaging and anatomical variation redefine flap design principles.
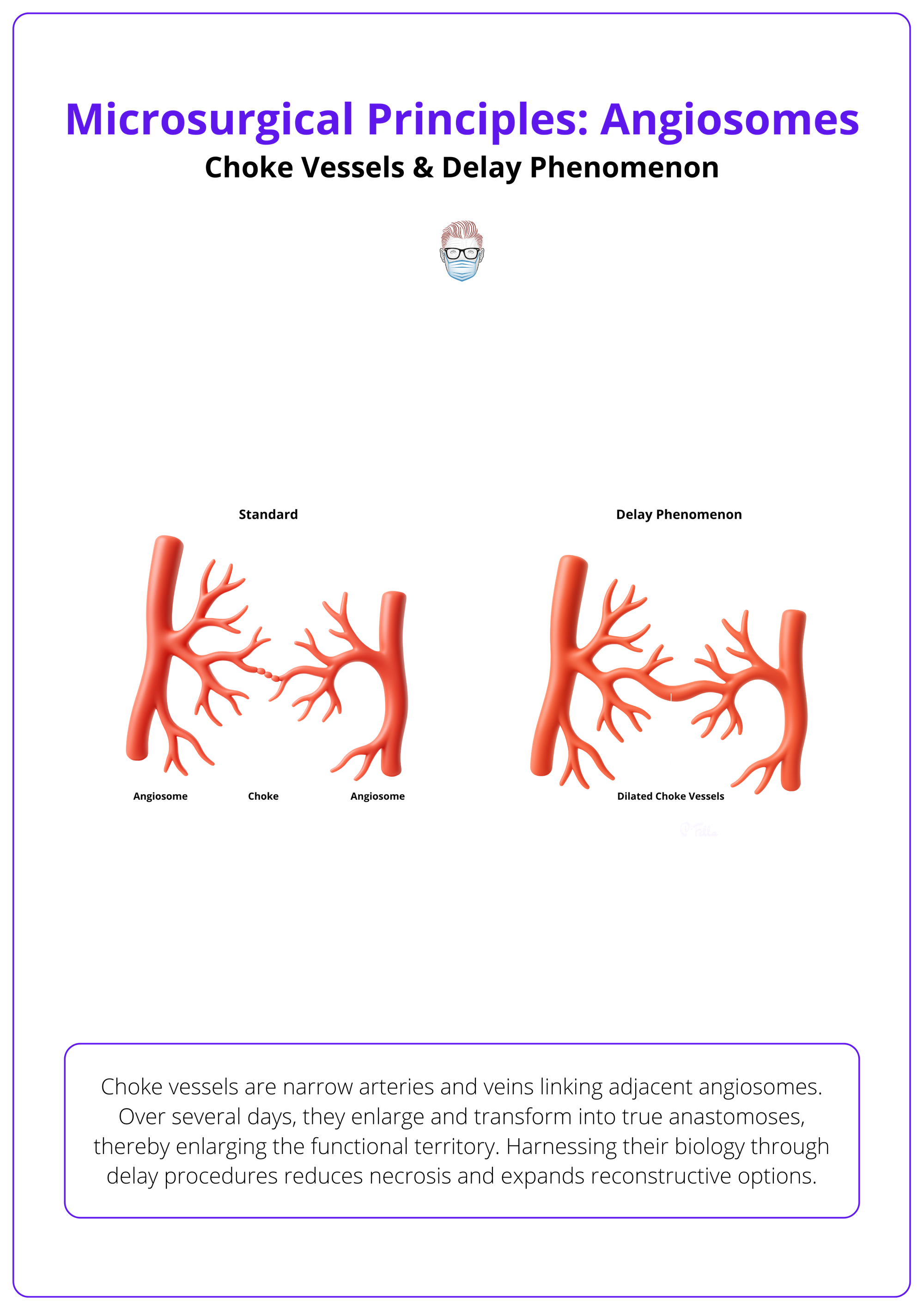
Choke Vessels and the Delay Phenomenon
Choke vessels are narrow arteries and veins linking adjacent angiosomes. Over several days, they enlarge and transform into true anastomoses, thereby enlarging the functional territory. Harnessing their biology through delay procedures reduces necrosis and expands reconstructive options.
Besides the main source vessels, flap survival depends on these interconnections. Initially small and low‑flow, choke vessels can be coaxed to dilate. Surgeons must know how long this takes, when to perform staged delay and where to place incisions.
Characteristics & Delay
- Morphology: Choke vessels are small arteries and veins connecting adjacent angiosomes. They have reduced calibre and minimal flow compared with true anastomoses.
- Growth Patterns: Their orientation and diameter reflect developmental growth patterns; arteries have fixed destinations but variable origins, while avalvular veins accompany them and allow bidirectional flow.
- Delay Phenomenon: When vascular supply is temporarily reduced (e.g., partial flap elevation), choke vessels dilate over 3-10 days, converting into true anastomoses. Delayed flaps exploit this process to increase viability.
Practical Guidance
- Plan for Dynamic and Potential Territories: Tissue beyond the anatomical zone may be included if enough time is allowed for choke vessels to open.
- Delay When Needed: If a flap design crosses into neighbouring angiosomes, perform a staged delay to enlarge the vascular network.
- Respect Vascular Borders: Place incisions along angiosome boundaries when both sides have good perfusion; include adjacent territories when one side is compromised.
Choke vessels and the delay phenomenon are illustrated below.
Clinical Applications of Angiosomes
Angiosome mapping informs every stage of flap surgery from design through perforator selection to intra‑operative decision‑making. Thereby improving outcomes and guiding targeted revascularisation.
In practice, surgeons pair defect analysis with donor‑site angiosome maps, choose the best perforators and decide whether delay, supermicrosurgery or other strategies are required. Imaging tools such as Doppler and ICG angiography provide dynamic confirmation that complements anatomical planning.
Flap Design & Selection
- Design Within Reliable Zones: Pedicled and perforator flaps should stay within well‑perfused zones; for example, discard or deepithelialise Zone IV of the DIEP flap and avoid overharvesting beyond radial forearm, scapular or facial artery angiosomes without delay.
Perforator Planning
- Identify Dominant Perforators: In the DIEP flap, medial‑row perforators perfuse central and contralateral tissue (Zones I-II) while lateral‑row perforators perfuse ipsilateral lateral tissue (Zones I-III).
- Tailor Flap Choice: For ALT, SGAP and similar flaps, use Doppler or CTA/ICG to locate perforators that supply the needed tissue and minimise donor morbidity.
Intra‑operative Strategy
- Place Incisions Wisely: Incisions should follow angiosome borders to maximise perfusion; when one angiosome is ischaemic, include adjacent territories to preserve blood supply.
- Use Delay When Needed: Stage flap elevation when crossing into a neighbouring angiosome; allow time for choke vessels to dilate.
- Consider Supermicrosurgery: When major recipient vessels are absent (e.g., diabetic foot), anastomose to small perforators from adjacent angiosomes.
Perfusion Assessment & Imaging
- Doppler Ultrasound: Locates perforators and arterial boundaries pre‑ and intra‑operatively.
- Indocyanine Green (ICG) Angiography: Provides real‑time perfusion maps and confirms viability of extended zones.
- Skin Perfusion Pressure (SPP): Helps identify dominant arteries in critical limb ischaemia.
- CT/MR Angiography: Offers detailed maps of perforators and reveals individual vascular variations.
Benefits and Limitations of the Angiosome Concept
Angiosome‑based planning reduces necrosis, improves aesthetics, and guides targeted revascularisation, yet its predictive value is limited by individual vascular variability, comorbidities, and the need for time‑dependent dilation.
The angiosome model has transformed reconstruction, but surgeons must apply it judiciously. Benefits include predictable perfusion and tailored interventions, while limitations relate to anatomical variation, patient factors and the dynamic behaviour of choke vessels. Supplemental imaging and clinical judgement remain essential.
Benefits
- Predictable Perfusion: Mapping angiosomes provides a framework for flap design, reducing distal necrosis (e.g., discarding Zone IV in abdominal flaps).
- Optimised Aesthetics and Function: Using tissue from the same angiosome achieves better colour, contour and reduced contraction compared with skin grafts.
- Targeted Revascularisation: Directing bypass or angioplasty to the artery feeding the affected angiosome improves wound healing in critical limb ischaemia.
- Perforator Planning: Matching perforators to zones ensures minimal donor morbidity and facilitates thin, pliable flaps.
Limitations
- Anatomical Variation: Individuals deviate from standard zone maps. Perfusion may differ based on perforator row.
- Time Dependence: Choke vessel dilation requires 4-10 days. Urgent reconstructions may not allow delay.
- Comorbidities: Diabetes, vascular disease and smoking impair perfusion and choke vessel maturation. Patient optimisation is vital.
- Need for Imaging: CT/CTA and ICG are often required to confirm perforator anatomy and perfusion patterns. Access and cost may limit use.
Common Complications & Mitigation
- Partial Flap or Fat Necrosis: Occurs when tissue beyond the perfused zone is included; prevent by adhering to zone maps and performing delay if needed.
- Venous Congestion: Results from pedicle kinking or tight closure. Prevent by positioning pedicles gently and ensuring tension‑free inset.
- Trapdoor Deformity: Caused by circular scars contracting. Avoid by designing flaps with angular shapes and placing incisions along relaxed skin tension lines.
- Poor Healing: Associated with comorbidities or compromised perfusion; optimise patient health, revascularise when necessary and monitor post‑operative perfusion.
Conclusion
1. Importance of Angiosomes: An angiosome is a three‑dimensional territory supplied by one artery and vein; using these maps reduces necrosis and guides revascularisation.
2. Principles & Mapping: Fundamental laws (equilibrium, continuity, and neurovascular relationships) and knowledge of perforator types underpin perforator‑flap surgery
3. Territory Stratification: Distinguish anatomical, dynamic, and potential territories; functional angiosomes may extend beyond anatomical boundaries when true anastomoses exist
4. Choke Vessels & Delay: Recognise that choke vessels take days to dilate; delay procedures expand functional territories and inform incision placement
5. Clinical Applications: Apply angiosome maps to design flaps, select perforators, plan incisions, use delay, and assess perfusion with Doppler or ICG.
6. Balanced Perspective: Leverage the benefits of angiosomes while acknowledging limitations related to vascular variability, comorbidities, and the need for imaging.
Further Reading
- Taylor GI, Palmer JH. The vascular territories (angiosomes) of the body: experimental study and clinical applications. British Journal of Plastic Surgery (1987). The seminal paper outlining the angiosome concept.
- Scheflan M, Hartrampf CR. Breast reconstruction with the transverse rectus abdominis myocutaneous (TRAM) flap. Annals of Plastic Surgery (1983). Describes the original four‑zone perfusion model for abdominal flaps.
- Holm C et al. Perfusion zones of the DIEP flap revisited. Plastic and Reconstructive Surgery (2006). Provides data demonstrating that lateral zones may out‑perfuse contralateral zones.
- Saint‑Cyr M et al. The perforasome theory: vascular anatomy and clinical implications. Clinical Plastic Surgery (2011). Introduces the perforasome concept and emphasises perforator‑row–dependent perfusion.
- Fujii M, Terashi H. Angiosome and Tissue Healing. Annals of Vascular Diseases (2019). Describes clinical applications of the angiosome concept in critical limb ischaemia and foot surgery.
- Attinger CE et al. Angiosome theory and foot surgery. Plastic and Reconstructive Surgery (1999). Applies the concept to safe incisions, flap planning and revascularisation in the diabetic foot.


