Summary Card
Overview
Velopharyngeal dysfunction (VPD) is the inability to close the velopharyngeal sphincter during speech, resulting in hypernasal speech, nasal air escape, poor oral pressure, and reduced intelligibility.
Relevant Anatomy
The velopharyngeal (VP) sphincter is a dynamic muscular valve made up of the soft palate (velum) and the lateral and posterior pharyngeal walls.
Aetiology
VPD arises from structural, neuromuscular, or functional causes. Structural causes, particularly cleft palate and post-surgical changes are the most common, but multiple overlapping factors may contribute.
Clinical Assessment
Assessment of VPD involves detailed perceptual speech analysis and imaging studies (nasoendoscopy and/or videofluoroscopy), ideally performed in a multidisciplinary setting.
Management
Successful VPD management requires a multidisciplinary approach. Surgical correction of structural deficits is often combined with speech therapy to restore normal speech while preserving airway function.
Primary Contributor: Dr Rob Browne.
Verified by thePlasticsFella ✅
Overview of Velopharyngeal Dysfunction
Velopharyngeal dysfunction (VPD) is the inability to close the velopharyngeal sphincter during speech, resulting in hypernasal speech, nasal air escape, poor oral pressure, and reduced intelligibility.
Early diagnosis and multidisciplinary treatment are essential, but outcomes remain variable despite optimal care.
VPD describes the clinical presentation of incomplete closure of the velopharyngeal sphincter, unlike older terms such as “incompetence,” “insufficiency,” or “mislearning”, which imply a specific cause. VPD is a neutral umbrella term that focuses on functional impairment, regardless of origin.¹
Functionally, VPD leads to,
- Hypernasal speech.
- Audible nasal airflow.
- Cleft-type articulation patterns.
- Reduced production of oral pressure consonants.
- Overall decreased speech intelligibility.
This can affect VPD is notoriously difficult to manage. Even with early access to multidisciplinary cleft teams (speech therapy, ENT, and surgery), outcomes can vary depending on the underlying cause, anatomy, and patient-specific factors.
Children with VPD often develop compensatory articulation patterns (e.g., glottal stops, pharyngeal fricatives) that persist even after surgical correction. Early speech therapy is critical to retrain correct articulation and maximize surgical outcomes.
Relevant Anatomy for Velopharyngeal Dysfunction
The velopharyngeal (VP) sphincter is a dynamic muscular valve made up of the soft palate (velum) and the lateral and posterior pharyngeal walls. Closure of this sphincter separates the oral and nasal cavities, and is essential for normal speech.
The velopharyngeal (VP) sphincter is a dynamic muscular valve that regulates airflow between the oral and nasal cavities. Its function is essential for producing normal speech and maintaining pressure for consonant articulation.
During speech, the soft palate elevates while the lateral and posterior pharyngeal walls constrict, creating a temporary seal that directs airflow and sound through the oral cavity. The sphincter also adjusts dynamically for nasal sounds and breathing.
Muscles Involved in VP Closure
- Levator veli palatini elevates the soft palate.
- Tensor veli palatini tenses the soft palate; opens the Eustachian tube.
- Musculus uvulae adds bulk and midline stiffening to the soft palate.
- Palatoglossus depresses the palate and elevates the tongue.
- Palatopharyngeus narrows the pharynx and aids in palatal movement.
- Superior constrictor assists in pharyngeal wall contraction.
Coordination During Speech
- Complete closure for most consonants and all vowels.
- Partial opening for nasal consonants (e.g., m, n, ng).
- Full opening during nasal breathing.
Innervation
- Pharyngeal plexus (CN X): Motor innervation to all VP muscles.
- Exception: Tensor veli palatini is innervated by the mandibular branch of the trigeminal nerve (CN V3).
Anatomy of the VP sphincter is illustrated below.
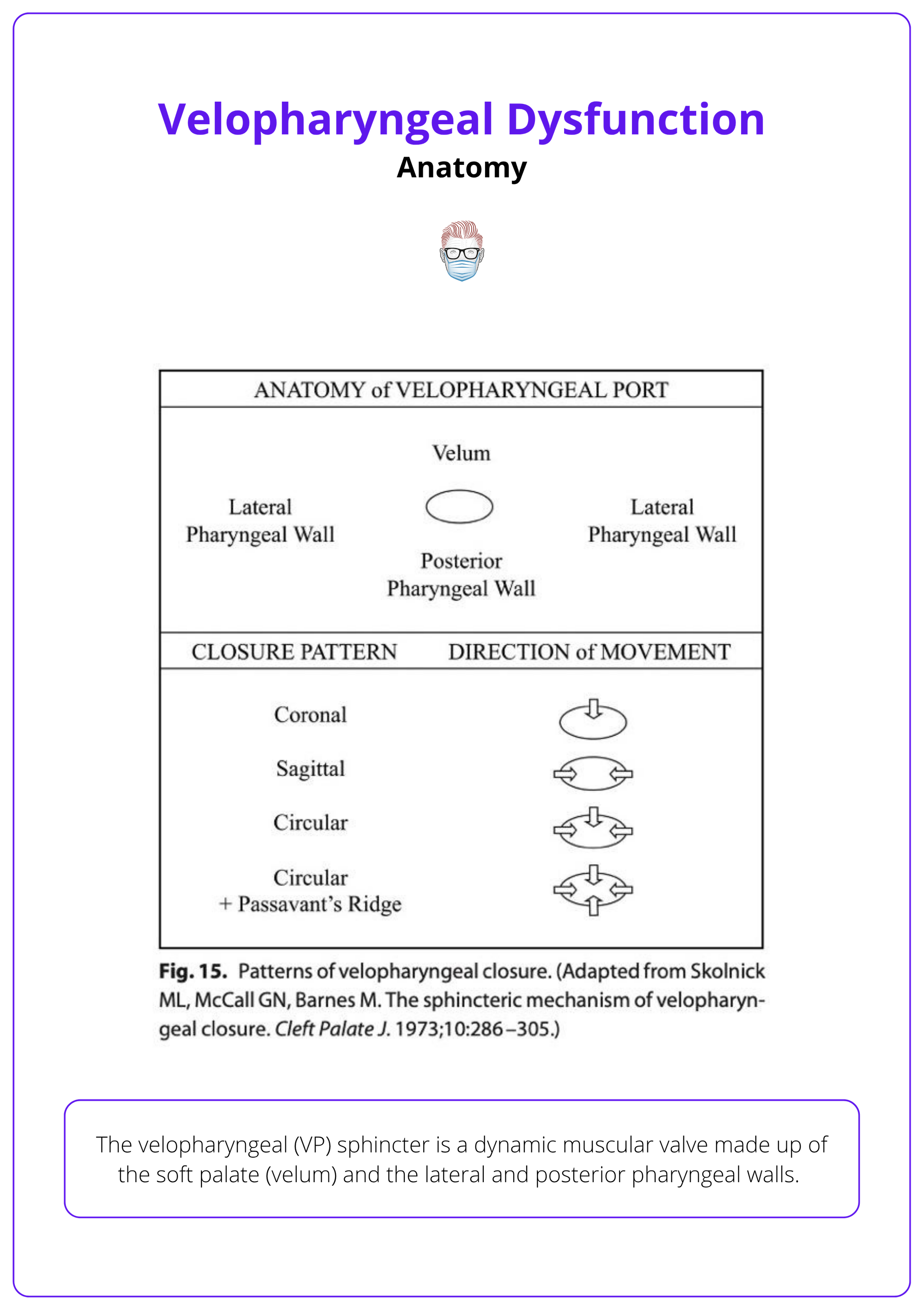
The levator veli palatini muscle forms a functional sling that elevates the soft palate. In cleft palate patients, this sling is often misoriented, running abnormally along the posterior hard palate instead of forming a proper loop, which is a major contributor to VPD.
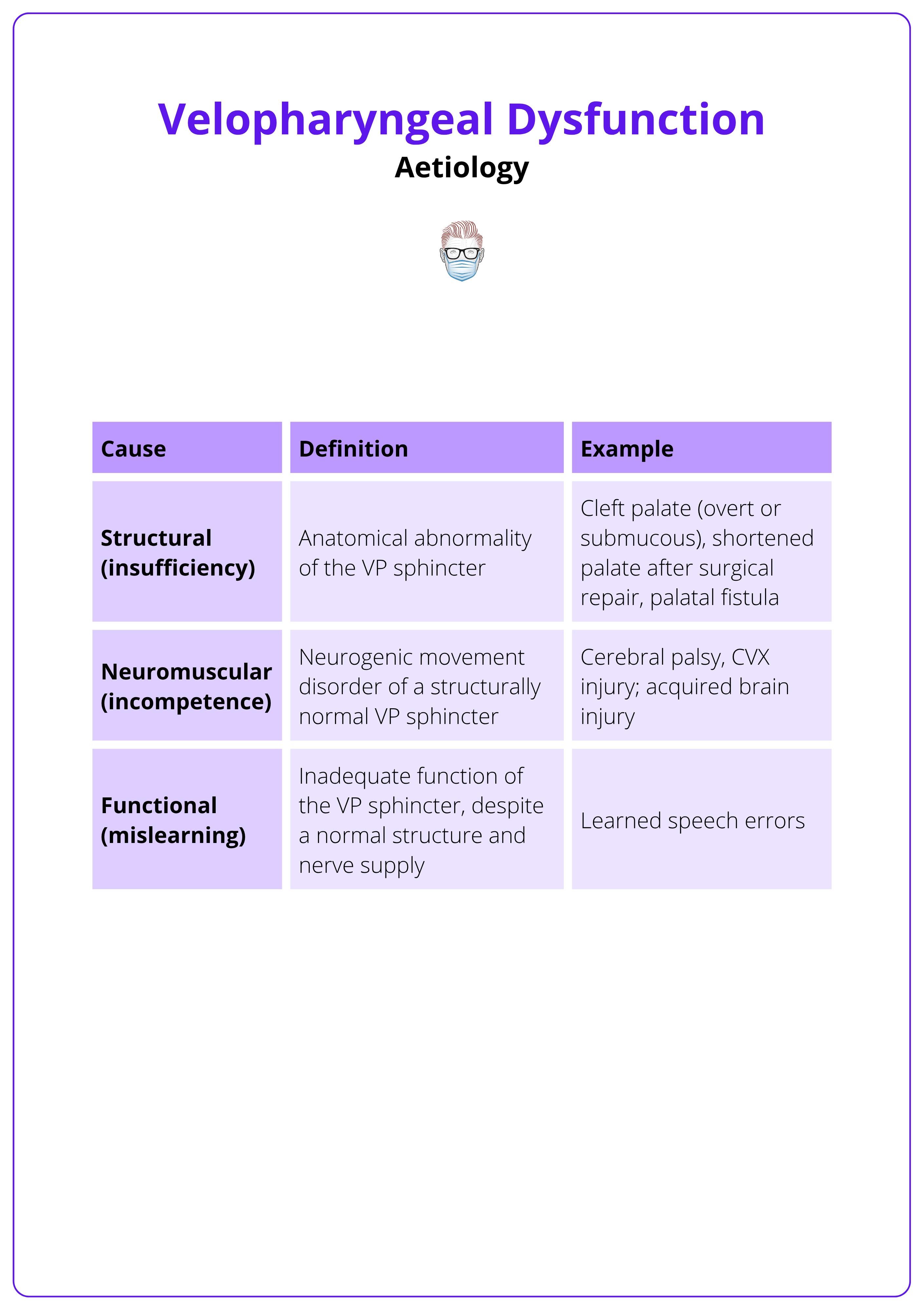
Aetiology of Velopharyngeal Dysfunction
VPD arises from structural, neuromuscular, or functional deficits. While structural causes, especially cleft palate and post-surgical changes are most common, many patients have overlapping contributions, particularly in syndromic conditions.
VPD Subtypes
- StructuralMost common cause, particularly after cleft palate repair.Post-op VPD rates vary (~20% across cleft types) due to,Surgical technique and experience.Severity of cleft (syndromic vs. non-syndromic).Palatal length and mobility.Presence of oronasal fistula.High-Risk Group: 22q11.2 deletion syndrome — VPD incidence up to 65% due to combined structural and neuromuscular deficits.
- NeuromuscularOften occurs in isolation or alongside syndromic clefts.Involves poor muscle tone or innervation affecting the levator sling or pharyngeal walls.Common in 22q11.2, cerebral palsy, and Moebius syndrome.
- Functional (Mislearning)Learned articulation errors despite structurally competent anatomy.Requires targeted speech therapy, not surgery.
VPD causes are broadly classified into three subtypes, as summarised below.
Pathophysiology in Cleft Palate
- Before Repair
- Levator veli palatini inserts abnormally into the posterior hard palate.
- Fails to form a functioning muscular sling.
- Results in poor velar elevation → VPD.
- After Repair
- Persistent VPD may result from,
- Shortened or scarred soft palate.
- Residual oronasal fistula.
- Hypoplastic adenoids (common in 22q11.2).
- Persistent VPD may result from,
Associated Conditions
- Cleft lip and palate (especially bilateral).
- Submucous cleft palate.
- 22q11.2 deletion syndrome.
- Van der Woude syndrome (IRF6 mutation).
- Other syndromic and neuromuscular disorders.
Risk Factors
Environmental/Maternal
- Smoking
- Diabetes
- Hypertension
- Obesity
- Teratogens: Steroids, Phenytoin
Post-Operative Risk Factors
- Patient-Related
- Large pre-op cleft width.
- Bilateral cleft lip and palate.
- Short palatal length.
- Surgical-Related
- Post-op palatal fistula.
Genetic
- 22q11.2 deletion syndrome.
- Van der Woude syndrome (IRF6 mutation).
Children with 22q11.2 deletion syndrome often require tailored VPD surgery due to dual deficits—structural (cleft or hypoplastic velum) and neuromuscular (poor lateral wall movement).
Clinical Assessment for Velopharyngeal Dysfunction
Assessment of VPD involves detailed perceptual speech analysis and imaging studies (nasoendoscopy and/or videofluoroscopy), ideally performed in a multidisciplinary setting.
Suspected VPD should be assessed at a specialist cleft/velopharyngeal dysfunction clinic by a team including surgeons, speech therapists, and other specialists.
Assessment components include,
- Comprehensive medical and developmental history.
- Physical examination of the palate.
- Perceptual speech analysis (initial and ongoing).
- Lateral video fluoroscopy and/or nasoendoscopy for dynamic assessment.
Perceptual speech analysis is the first and most essential diagnostic step. Children with hypernasal speech but no cleft history may have a submucous cleft, neuromuscular condition, or syndrome.
Ongoing Follow-up in Cleft Palate Patients
- Post-cleft palate repair, children require regular speech evaluations.
- Monitoring should continue into adolescence.
Perceptual Speech Analysis – Key VPD Features
- Resonance: Tonal speech quality – hypernasal speech is common in VPD (low intensity, muffled).
- Nasal Airflow: Turbulence and/or emission.
- Cleft Type Articulation Characteristics: Obligatory distortions – corrected with surgery.
- Decreased oral pressure consonant production (e.g., inability to say p in “pop”).
Physical Examination
Focuses on identifying,
- Submucous cleft
- Neuromuscular signs
- Syndromic features
Other relevant features to document include,
- Occlusion and dentition
- Presence/size of oronasal fistula
- Tonsillar/adenoidal hypertrophy
Imaging Studies
- Lateral Video Fluoroscopy
- Dynamic x-ray of speech using barium contrast.
- Measures VP gap size.
- Does not assess closure pattern.
- Some centres use this as the sole imaging modality (esp. in Europe).
- Radiation exposure is a limitation.
- Nasoendoscopy
- Direct visualisation of VP sphincter function during speech.
- Useful in identifying closure patterns, which may inform surgical planning.
- May be poorly tolerated in young children.
The image below summarises the closure patterns and VP wall motions.

Passavant’s ridge is a bulge in the posterior pharyngeal wall, formed.
Passavant’s ridge is a bulge of the posterior pharyngeal wall from the superior constrictor muscle that narrows the VP gap. Not present in all patients and functionally variable.
Other Tools (Less Common)
- Nasometry: Objective acoustic analysis of nasality.
- MRI: Offers anatomical detail but is less widely used due to limited dynamic function imaging.
Management of Velopharyngeal Dysfunction
Successful VPD management requires a multidisciplinary approach. Surgical correction of structural deficits is often combined with speech therapy to restore normal speech while preserving airway function.
Management is tailored individually and typically involves,
- Cleft/craniofacial surgeon.
- Speech and language therapist.
- ENT or audiology input, if required.
Structural problems require surgery; speech therapy alone is insufficient.
Conversely, speech therapy is essential for functional recovery postoperatively.
Surgical Goals
- Restore normal speech without compromising airway patency.
- Enable controlled separation of the oral and nasal cavities during speech.
Surgical Approaches
There are three main surgical strategies.
Some surgeons tailor their surgical technique based on nasoendoscopy closure pattern, though in practice, many rely on personal experience.
Palatoplasty Procedures
- Focus: Lengthen the soft palate and reposition velar muscles.
- Principles
- Tension-free closure.
- Three-layered closure (nasal mucosa, muscle, oral mucosa),
- Preservation of palatal mobility.
- Techniques
- Furlow Double Opposing Z-Plasty
- Described in cleft palate section.
- Z-plasties reorient muscle fibres and lengthen the velum.
- Intravelar Veloplasty (IVV)
- Muscle dissection and reorientation, described by Sommerlad.
- Useful in restoring the levator sling.
- Buccal Myomucosal Flaps
- Indication: Short or scarred palates with moderate-to-large gaps.
- Technique: Soft palate is freed and repositioned posteriorly; buccal flaps are used to fill the resulting defect.
- Benefits: Preserves velar mobility.
- Limitations: May need a second stage to divide flap pedicle if speech/feeding are affected.
- Furlow Double Opposing Z-Plasty
Pharyngoplasty Procedures
- Pharyngeal Flap
- Indications: Long velum with inconsistent or weak movement (e.g., 22q11 syndrome).
- Superiorly based flap from the posterior pharyngeal wall.
- Inserted into the soft palate to narrow midline defect.
- Allows lateral airflow; often preferred in non-cleft VPD.
- Benefits: Good speech outcomes.
- Limitations: Low revision rate.
- Sphincter Pharyngoplasty
- Indications: Lateral wall dysfunction/lateral gaps.
- Bilateral palatopharyngeus flaps are inset transversely.
- Designed for lateral closure defects.
- Benefits: More dynamic closure using palatopharyngeus muscles.
- Limitation: Increased risk of obstructive sleep apnoea (OSA).
Posterior Pharyngeal Wall Augmentation
- Technique: Autologous (fat/cartilage) or alloplastic (e.g., silicone) material is implanted behind the posterior pharyngeal mucosa.
- Indications
- Small residual VP gaps.
- To supplement other procedures (e.g., pharyngoplasty).
- Benefits: Minimal airway obstruction risk.
- Limitation: Less effective for larger VP gaps.
Outcomes & Considerations
- All procedures improve VPD features, but not all eliminate them.
- Speech improvements are gradual.
- Noticeable Change: ~12 months post-op.
- Further improvements can occur for 3–4 years.
- OSA risk is highest with pharyngeal flap and sphincter pharyngoplasty. Lower with palatoplasty or augmentation.
Conclusion
1. Overview: VPD is the failure of velopharyngeal closure during speech, leading to hypernasality, nasal air escape, and poor speech intelligibility.
2. Anatomy: The VP sphincter is formed by the soft palate and pharyngeal walls, closing dynamically during speech via CN X-innervated muscles (except the tensor via CN V3).
3. Aetiology: Causes include structural (e.g., cleft palate), neuromuscular, or functional deficits — often overlapping; cleft repair remains the most common contributor.
4. Assessment: Diagnosis involves speech analysis, physical exam, and imaging (nasoendoscopy or videofluoroscopy); multidisciplinary input is essential.
5. Management: Treatment combines surgery and speech therapy. Surgical options include palatoplasty, pharyngoplasty, and augmentation, tailored to gap type.
6. Outcomes: Speech improves gradually post-op (12+ months); success varies by anatomy and cause. Pharyngeal flaps and sphincteroplasty carry higher OSA risk.
Further Reading
- Woo AS. Velopharyngeal dysfunction. Semin Plast Surg. Nov 2012;26(4):170-7. doi:10.1055/s-0033-1333882
- Kummer AW. Speech evaluation for patients with cleft palate. Clin Plast Surg. Apr 2014;41(2):241-51. doi:10.1016/j.cps.2013.12.004
- Sainsbury DCG, Williams CC, Butterworth S, et al. Patient Factors Influencing Speech Outcomes in Velopharyngeal Function Following Initial Cleft Palate Repair: A Systematic Review and Meta-Analysis. The Cleft Palate Craniofacial Journal. 2024;61(12):2022-2037. doi:10.1177/10556656231191384
- McDonald-McGinn DM, Sullivan KE, Marino B, et al. 22q11.2 deletion syndrome. Nat Rev Dis Primers. Nov 19 2015;1:15071. doi:10.1038/nrdp.2015.71
- Sweeney WM, Lanier ST, Purnell CA, Gosain AK. Genetics of Cleft Palate and Velopharyngeal Insufficiency. J Pediatr Genet. Mar 2015;4(1):9-16. doi:10.1055/s-0035-1554978
- Ács M, Cavalcante BGN, Bănărescu M, et al. Maternal factors increase risk of orofacial cleft: a meta-analysis. Scientific Reports. 2024/11/15 2024;14(1):28104. doi:10.1038/s41598-024-79346-7
- Garland K, Dworschak-Stokan A, Matic D. Patient and surgical factors that affect the development of velopharyngeal insufficiency. J Plast Reconstr Aesthet Surg. Oct 2022;75(10):3813-3816. doi:10.1016/j.bjps.2022.06.036
- Perry JL, Kuehn DP, Sutton BP, Fang X. Velopharyngeal Structural and Functional Assessment of Speech in Young Children Using Dynamic Magnetic Resonance Imaging. Cleft Palate Craniofac J. Jul 2017;54(4):408-422. doi:10.1597/15-120


